Fatty liver disease
Parts of this article (those related to the use of the new 2023 nomenclature) need to be updated. (November 2023) |
| Fatty liver | |
|---|---|
| Other names | Hepatic steatosis |
 | |
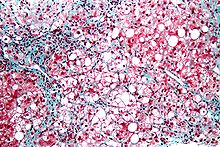
| Micrograph showing a fatty liver (macrovesicular steatosis), as seen in non-alcoholic fatty liver disease. Trichrome stain. | |
| Specialty | Gastroenterology |
| Symptoms | None, tiredness, pain in the upper right side of the abdomen[1][2] |
| Complications | Cirrhosis, liver cancer, esophageal varices[1][3] |
| Types | Non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease[1] |
| Causes | Alcohol, diabetes, obesity[3][1] |
| Diagnostic method | Based on the medical history supported by blood tests, medical imaging, liver biopsy[1] |
| Differential diagnosis | Viral hepatitis, Wilson disease, primary sclerosing cholangitis[3] |
| Treatment | Avoiding alcohol, weight loss[3][1] |
| Prognosis | Good if treated early[3] |
| Frequency | NAFLD: 30% (Western countries)[2] ALD: >90% of heavy drinkers[4] |
Fatty liver disease (FLD), also known as hepatic steatosis and steatotic liver disease (SLD), is a condition where excess fat builds up in the liver.[1] Often there are no or few symptoms.[1][2] Occasionally there may be tiredness or pain in the upper right side of the abdomen.[1] Complications may include cirrhosis, liver cancer, and esophageal varices.[1][3]
The main subtypes of fatty liver disease are metabolic dysfunction–associated steatotic liver disease (MASLD, formerly "non-alcoholic fatty liver disease" (NAFLD)) and alcohol-associated liver disease (ALD), with the category "metabolic and alcohol associated liver disease" (metALD) describing an overlap of the two.[5]
The primary risks include alcohol, type 2 diabetes, and obesity.[1][3] Other risk factors include certain medications such as glucocorticoids, and hepatitis C.[1] It is unclear why some people with NAFLD develop simple fatty liver and others develop nonalcoholic steatohepatitis (NASH), which is associated with poorer outcomes.[1] Diagnosis is based on the medical history supported by blood tests, medical imaging, and occasionally liver biopsy.[1]
Treatment of NAFLD is generally by dietary changes and exercise to bring about weight loss.[1] In those who are severely affected, liver transplantation may be an option.[1] More than 90% of heavy drinkers develop fatty liver while about 25% develop the more severe alcoholic hepatitis.[4] NAFLD affects about 30% of people in Western countries and 10% of people in Asia.[2] NAFLD affects about 10% of children in the United States.[1] It occurs more often in older people and males.[3][6]
Classification
[edit]Fatty liver disease was classified into:
In 2023, a new nomenclature was chosen,[5][7] with the classifications including:
- Metabolic dysfunction–associated steatotic liver disease (MASLD), including:
- Metabolic and alcohol associated liver disease (metALD). Describes those with MASLD who consume greater amounts of alcohol per week but not enough to be categorized as ALD)
- Alcohol-associated liver disease (ALD)
- Specific aetiology SLD (including drug-induced, monogenic diseases and others)
Signs and symptoms
[edit]Often there are no or few symptoms.[1] Occasionally there may be tiredness or pain in the upper right side of the abdomen.[1]
Complications
[edit]Fatty liver can develop into hepatic fibrosis, cirrhosis or liver cancer.[8] For people affected by NAFLD, the 10-year survival rate was about 80%. The rate of progression of fibrosis is estimated to be one per 7 years in NASH and one per 14 years in NAFLD, with an increasing speed.[9][10] There is a strong relationship between these pathologies and metabolic illnesses (diabetes type II, metabolic syndrome). These pathologies can also affect non-obese people, who are then at a higher risk.[8]
Less than 10% of people with cirrhotic alcoholic FLD will develop hepatocellular carcinoma,[11] the most common type of primary liver cancer in adults, but up to 45% people with NASH without cirrhosis can develop hepatocellular carcinoma.[12]
The condition is also associated with other diseases that influence fat metabolism.[13]
Causes
[edit]
Fatty liver (FL) is commonly associated with metabolic syndrome (diabetes, hypertension, obesity, and dyslipidemia), but can also be due to any one of many causes:[14][15]
- Alcohol
- Alcohol use disorder is one of the causes of fatty liver due to production of toxic metabolites like aldehydes during metabolism of alcohol in the liver. This phenomenon most commonly occurs with chronic alcohol use disorder.
- Metabolic
- abetalipoproteinemia, glycogen storage diseases, Weber–Christian disease, acute fatty liver of pregnancy, lipodystrophy
- Nutritional
- obesity, malnutrition, total parenteral nutrition, severe weight loss, refeeding syndrome, jejunoileal bypass, gastric bypass, jejunal diverticulosis with bacterial overgrowth
- Drugs and toxins
- amiodarone, methotrexate, diltiazem, expired tetracycline, highly active antiretroviral therapy, glucocorticoids, tamoxifen,[16] environmental hepatotoxins (e.g., phosphorus, mushroom poisoning)
- Other
- celiac disease,[17] inflammatory bowel disease, HIV, hepatitis C (especially genotype 3), and alpha 1-antitrypsin deficiency[18]
Pathology
[edit]
The fatty change represents the intracytoplasmatic accumulation of triglycerides (neutral fats). At the beginning, the hepatocytes present small fat vacuoles (liposomes) around the nucleus (microvesicular fatty change). In this stage, liver cells are filled with multiple fat droplets that do not displace the centrally located nucleus. In the late stages, the size of the vacuoles increases, pushing the nucleus to the periphery of the cell, giving a characteristic signet ring appearance (macrovesicular fatty change). These vesicles are well-delineated and optically "empty" because fats dissolve during tissue processing. Large vacuoles may coalesce and produce fatty cysts, which are irreversible lesions. Macrovesicular steatosis is the most common form and is typically associated with alcohol, diabetes, obesity, and corticosteroids. Acute fatty liver of pregnancy and Reye's syndrome are examples of severe liver disease caused by microvesicular fatty change.[19] The diagnosis of steatosis is made when fat in the liver exceeds 5–10% by weight.[13][20][21]
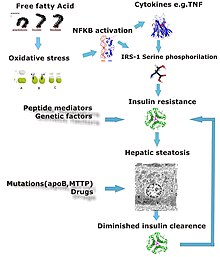
Defects in fatty acid metabolism are responsible for pathogenesis of FLD, which may be due to imbalance in energy consumption and its combustion, resulting in lipid storage, or can be a consequence of peripheral resistance to insulin, whereby the transport of fatty acids from adipose tissue to the liver is increased.[13][22] Impairment or inhibition of receptor molecules (PPAR-α, PPAR-γ and SREBP1) that control the enzymes responsible for the oxidation and synthesis of fatty acids appears to contribute to fat accumulation. In addition, alcohol use disorder is known to damage mitochondria and other cellular structures, further impairing cellular energy mechanism. On the other hand, non-alcoholic FLD may begin as excess of unmetabolised energy in liver cells. Hepatic steatosis is considered reversible and to some extent nonprogressive if the underlying cause is reduced or removed.
Severe fatty liver is sometimes accompanied by inflammation, a situation referred to as steatohepatitis. Progression to alcoholic steatohepatitis (ASH) or non-alcoholic steatohepatitis (NASH) depends on the persistence or severity of the inciting cause. Pathological lesions in both conditions are similar. However, the extent of inflammatory response varies widely and does not always correlate with degree of fat accumulation. Steatosis (retention of lipid) and onset of steatohepatitis may represent successive stages in FLD progression.[23]
Liver disease with extensive inflammation and a high degree of steatosis often progresses to more severe forms of the disease.[24] Hepatocyte ballooning and necrosis of varying degrees are often present at this stage. Liver cell death and inflammatory responses lead to the activation of hepatic stellate cells, which play a pivotal role in hepatic fibrosis. The extent of fibrosis varies widely. Perisinusoidal fibrosis is most common, especially in adults, and predominates in zone 3 around the terminal hepatic veins.[25]
The progression to cirrhosis may be influenced by the amount of fat and degree of steatohepatitis and by a variety of other sensitizing factors. In alcoholic FLD, the transition to cirrhosis related to continued alcohol consumption is well-documented, but the process involved in non-alcoholic FLD is less clear.
Diagnosis
[edit]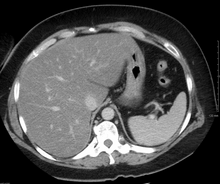
| Flow chart for diagnosis[15] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ‡ Criteria for nonalcoholic fatty liver disease: consumption of ethanol less than 20 g/day for women and 30 g/day for men[26] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Most individuals are asymptomatic and are usually discovered incidentally because of abnormal liver function tests or hepatomegaly noted in unrelated medical conditions. Elevated liver enzymes are found in as many as 50% of patients with simple steatosis.[27]: 1794 The serum alanine transaminase (ALT) level usually is greater than the aspartate transaminase (AST) level in the nonalcoholic variant and the opposite in alcoholic FLD (AST:ALT more than 2:1). Simple blood tests may help to determine the magnitude of the disease by assessing the degree of liver fibrosis.[28] For example, AST-to-platelets ratio index (APRI score) and several other scores, calculated from the results of blood tests, can detect the degree of liver fibrosis and predict the future formation of liver cancer.[29]
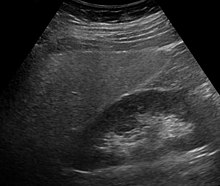
Imaging studies are often obtained during the evaluation process. Ultrasonography reveals a "bright" liver with increased echogenicity. Pocket-sized ultrasound devices might be used as point-of-care screening tools to diagnose liver steatosis.[30][31] Medical imaging can aid in diagnosis of fatty liver; fatty livers have lower density than spleens on computed tomography (CT), and fat appears bright in T1-weighted magnetic resonance images (MRIs). Magnetic resonance elastography, a variant of magnetic resonance imaging, is investigated as a non-invasive method to diagnose fibrosis progression.[32] Online calculators have been developed to assist in evaluating hepatic steatosis using CT and MRI findings. [33] Histological diagnosis by liver biopsy is the most accurate measure of fibrosis and liver fat progression as of 2018.[8] Conventional imaging methods, such as ultrasound, CT and MRI, are not specific enough to detect fatty liver disease unless fat occupies at least 30% of the liver volume.[34]
Treatment
[edit]Decreasing caloric intake by at least 30% or by approximately 750–1,000 kcal/day results in improvement in hepatic steatosis.[8] For people with NAFLD or NASH, weight loss via a combination of diet and exercise was shown to improve or resolve the disease.[8] In more serious cases, medications that decrease insulin resistance, hyperlipidemia, and those that induce weight loss such as bariatric surgery as well as vitamin E have been shown to improve or resolve liver function.[8][15]
Bariatric surgery, while not recommended in 2017 as a treatment for FLD alone, has been shown to revert FLD, NAFLD, NASH and advanced steatohepatitis in over 90% of people who have undergone this surgery for the treatment of obesity.[8][35]
In the case of long-term total-parenteral-nutrition-induced fatty liver disease, choline has been shown to alleviate symptoms.[36][37][38] This may be due to a deficiency in the methionine cycle.[39]
Epidemiology
[edit]NAFLD affects about 30% of people in Western countries and 10% of people in Asia.[2] In the United States, rates are around 35% with about 7% having the severe form NASH.[1] NAFLD affects about 10% of children in the United States.[1] Recently the term Metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed to replace NAFLD. MAFLD is a more inclusionary diagnostic name as it is based on the detection of fatty liver by histology (biopsy), medical imaging or blood biomarkers but should be accompanied by either overweight/obesity, type 2 diabetes mellitus, or metabolic dysregulation.[40] The new definition no longer excludes alcohol consumption or coexistence of other liver diseases such as viral hepatitis. Using this more inclusive definition, the global prevalence of MAFLD is an astonishingly high 50.7%.[40] Indeed, also using the old NAFLD definition, the disease is observed in up to 80% of obese people, 35% of whom progress to NASH,[41] and in up to 20% of normal weight people,[10] despite no evidence of excessive alcohol consumption. FLD is the most common cause of abnormal liver function tests in the United States.[14] Fatty liver is more prevalent in Hispanic people than white, with black people having the lowest prevalence.[10]
In the study Children of the 90s, 2.5% born in 1991 and 1992 were found by ultrasound at the age of 18 to have non-alcoholic fatty liver disease; five years later transient elastography found over 20% to have the fatty deposits on the liver, indicating non-alcoholic fatty liver disease; half of those were classified as severe. The scans also found that 2.4% had a degree of liver fibrosis, which can lead to cirrhosis.[42][43]
After the lockdown of the COVID-19 pandemic, a study demonstrated that 48% of patients with liver steatosis gained weight, while 16% had a worsened steatosis grade. Weight gain was associated with poor adherence to the suggested diet, reduced levels of physical activity, and increased prevalence of homozygosity for the PNPLA3 rs738409 single nucleotide polymorphism.[44] PNPLA3 rs738409 is already a known risk factor for NAFLD.[45][46]
Research
[edit]A systematic review and meta-analysis, published in 2024, found that growth hormone therapy may help in the management of fatty liver disease.[47]
In animals
[edit]Fatty liver disease can occur in pets such as reptiles (particularly turtles[48]) and birds[49] as well as mammals like cats and dogs.[50] The most common cause is overnutrition. A distinct sign in birds is a misshapen beak. Fatty livers can be induced via gavage in geese or ducks to produce foie gras. Fatty liver can also be induced in ruminants such as sheep by a high-caloric diet.[51][52]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w "Nonalcoholic Fatty Liver Disease & NASH". National Institute of Diabetes and Digestive and Kidney Diseases. November 2016. Retrieved 7 November 2018.
- ^ a b c d e Singh S, Osna NA, Kharbanda KK (28 September 2017). "Treatment options for alcoholic and non-alcoholic fatty liver disease: A review". World Journal of Gastroenterology. 23 (36): 6549–6570. doi:10.3748/wjg.v23.i36.6549. PMC 5643281. PMID 29085205.
- ^ a b c d e f g h Antunes C, Azadfard M, Hoilat GJ, Gupta M (2022). "Fatty Liver". StatPearls. StatPearls Publishing. PMID 28723021.
- ^ a b Basra S (2011). "Definition, epidemiology and magnitude of alcoholic hepatitis". World Journal of Hepatology. 3 (5): 108–113. doi:10.4254/wjh.v3.i5.108. PMC 3124876. PMID 21731902.
- ^ a b Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. (2023). "A multi-society Delphi consensus statement on new fatty liver disease nomenclature". Hepatology. 78 (6): 1966–1986. doi:10.1097/HEP.0000000000000520. hdl:10807/245116. ISSN 0270-9139. PMC 10653297. PMID 37363821. S2CID 259260747.
- ^ a b Iser D, Ryan M (July 2013). "Fatty liver disease—a practical guide for GPs". Australian Family Physician. 42 (7): 444–7. PMID 23826593.
- ^ "A Liver Disease Gets a New Name, Diagnostic Criteria". Medscape. Retrieved 4 September 2023.
- ^ a b c d e f g Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. (January 2018). "The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases". Hepatology. 67 (1): 328–357. doi:10.1002/hep.29367. hdl:1805/14037. PMID 28714183.
- ^ Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R (April 2015). "Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies". Clinical Gastroenterology and Hepatology. 13 (4): 643–654.e9. doi:10.1016/j.cgh.2014.04.014. PMC 4208976. PMID 24768810.
- ^ a b c Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. (20 September 2017). "Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention". Nature Reviews Gastroenterology & Hepatology. 15 (1): 11–20. doi:10.1038/nrgastro.2017.109. hdl:2318/1659230. PMID 28930295. S2CID 31345431.
- ^ Qian Y, Fan JG (May 2005). "Obesity, fatty liver and liver cancer". Hepatobiliary & Pancreatic Diseases International. 4 (2): 173–7. PMID 15908310.
- ^ Bellentani S (January 2017). "The epidemiology of non-alcoholic fatty liver disease". Liver International. 37: 81–84. doi:10.1111/liv.13299. PMID 28052624.
- ^ a b c Reddy JK, Rao MS (May 2006). "Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation". American Journal of Physiology. Gastrointestinal and Liver Physiology. 290 (5): G852-8. doi:10.1152/ajpgi.00521.2005. PMID 16603729.
- ^ a b Angulo P (18 April 2002). "Nonalcoholic Fatty Liver Disease". New England Journal of Medicine. 346 (16): 1221–1231. doi:10.1056/NEJMra011775. PMID 11961152.
- ^ a b c Bayard M, Holt J, Boroughs E (June 2006). "Nonalcoholic fatty liver disease". American Family Physician. 73 (11): 1961–8. PMID 16770927.
- ^ Osman KA, Osman MM, Ahmed MH (January 2007). "Tamoxifen-induced non-alcoholic steatohepatitis: where are we now and where are we going?". Expert Opinion on Drug Safety. 6 (1): 1–4. doi:10.1517/14740338.6.1.1. PMID 17181445. S2CID 33505288.
- ^ Marciano F, Savoia M, Vajro P (February 2016). "Celiac disease-related hepatic injury: Insights into associated conditions and underlying pathomechanisms". Digestive and Liver Disease. 48 (2): 112–9. doi:10.1016/j.dld.2015.11.013. PMID 26711682.
- ^ Valenti L, Dongiovanni P, Piperno A, Fracanzani AL, Maggioni M, Rametta R, et al. (October 2006). "Alpha 1-antitrypsin mutations in NAFLD: high prevalence and association with altered iron metabolism but not with liver damage". Hepatology. 44 (4): 857–64. doi:10.1002/hep.21329. PMID 17006922. S2CID 26068505.
- ^ Goldman L (2003). Cecil Textbook of Medicine – 2-Volume Set, Text with Continually Updated Online Reference. Philadelphia: W.B. Saunders Company. ISBN 978-0-7216-4563-6.[page needed]
- ^ Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. (July 2005). "The natural history of nonalcoholic fatty liver disease: a population-based cohort study". Gastroenterology. 129 (1): 113–21. doi:10.1053/j.gastro.2005.04.014. PMID 16012941.
- ^ Crabb DW, Galli A, Fischer M, You M (August 2004). "Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha". Alcohol. 34 (1): 35–8. doi:10.1016/j.alcohol.2004.07.005. PMID 15670663.
- ^ Medina J, Fernández-Salazar LI, García-Buey L, Moreno-Otero R (August 2004). "Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis". Diabetes Care. 27 (8): 2057–66. doi:10.2337/diacare.27.8.2057. PMID 15277442.
- ^ Day CP, James OF (April 1998). "Steatohepatitis: a tale of two "hits"?". Gastroenterology. 114 (4): 842–5. doi:10.1016/S0016-5085(98)70599-2. PMID 9547102.
- ^ Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM (February 2004). "Pathologic features associated with fibrosis in nonalcoholic fatty liver disease". Human Pathology. 35 (2): 196–9. doi:10.1016/j.humpath.2003.09.018. PMID 14991537.
- ^ Zafrani ES (January 2004). "Non-alcoholic fatty liver disease: an emerging pathological spectrum". Virchows Archiv. 444 (1): 3–12. doi:10.1007/s00428-003-0943-7. PMID 14685853. S2CID 7708476.
- ^ Adams LA, Angulo P, Lindor KD (29 March 2005). "Nonalcoholic fatty liver disease". Canadian Medical Association Journal. 172 (7): 899–905. doi:10.1503/cmaj.045232. PMC 554876. PMID 15795412.
- ^ Reid AE (2006). "Chapter 82: Nonalcoholic Fatty Liver Disease". In Feldman M, Friedman LS, Brandt LJ (eds.). Sleisenger and Fordtran's Gastrointestinal and Liver Disease (8th ed.). Philadelphia: W.B. Saunders Company. ISBN 978-1-4160-0245-1. Retrieved 4 July 2023 – via Internet Archive.
- ^ Peleg N, Issachar A, Sneh-Arbib O, Shlomai A (October 2017). "AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease". Digestive and Liver Disease. 49 (10): 1133–1138. doi:10.1016/j.dld.2017.05.002. PMID 28572039.
- ^ Peleg N, Sneh Arbib O, Issachar A, Cohen-Naftaly M, Braun M, Shlomai A (14 August 2018). "Noninvasive scoring systems predict hepatic and extra-hepatic cancers in patients with nonalcoholic fatty liver disease". PLOS ONE. 13 (8): e0202393. Bibcode:2018PLoSO..1302393P. doi:10.1371/journal.pone.0202393. PMC 6091950. PMID 30106985.
- ^ Miles DA, Levi CS, Uhanova J, Cuvelier S, Hawkins K, Minuk GY. Pocket-Sized Versus Conventional Ultrasound for Detecting Fatty Infiltration of the Liver. Dig Dis Sci. 2020 Jan;65(1):82-85. doi: 10.1007/s10620-019-05752-x. Epub 2019 Aug 2. PMID 31376083.
- ^ Costantino A, Piagnani A, Caccia R, Sorge A, Maggioni M, Perbellini R, Donato F, D'Ambrosio R, Sed NPO, Valenti L, Prati D, Vecchi M, Lampertico P, Fraquelli M. Reproducibility and accuracy of a pocket-size ultrasound device in assessing liver steatosis. Dig Liver Dis. 2023 Nov 27:S1590-8658(23)01032-0. doi: 10.1016/j.dld.2023.11.014. Epub ahead of print. PMID 38016894.
- ^ Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. (28 August 2015). "Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis". European Radiology. 26 (5): 1431–1440. doi:10.1007/s00330-015-3949-z. PMC 5051267. PMID 26314479.
- ^ "Liver Steatosis Calculator & Report Generator (CT & MRI)". Rad At Hand. Retrieved 26 December 2024.
- ^ Benedict M, Zhang X (June 2017). "Non-alcoholic fatty liver disease: An expanded review". World Journal of Hepatology. 9 (16): 715–732. doi:10.4254/wjh.v9.i16.715. PMC 5468341. PMID 28652891.
- ^ Fatty Liver at eMedicine
- ^ Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, et al. (November 1995). "Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation". Hepatology. 22 (5): 1399–403. doi:10.1002/hep.1840220510. PMID 7590654. S2CID 20227016.
- ^ Buchman AL, Dubin M, Jenden D, Moukarzel A, Roch MH, Rice K, et al. (April 1992). "Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients". Gastroenterology. 102 (4 Pt 1): 1363–70. doi:10.1016/0016-5085(92)70034-9. PMID 1551541.
- ^ Buchman AL, Ament ME, Sohel M, Dubin M, Jenden DJ, Roch M, et al. (2016). "Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial". Journal of Parenteral and Enteral Nutrition. 25 (5): 260–8. doi:10.1177/0148607101025005260. PMID 11531217.
- ^ Hollenbeck CB (August 2010). "The importance of being choline". Journal of the American Dietetic Association. 110 (8): 1162–5. doi:10.1016/j.jada.2010.05.012. PMID 20656090.
- ^ a b Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, et al. (February 2021). "Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults". Clinical Gastroenterology and Hepatology. 20 (3): e573 – e582. doi:10.1016/j.cgh.2021.02.030. PMID 33618024. S2CID 232018678.
- ^ Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. (15 November 2005). "The Metabolic Syndrome as a Predictor of Nonalcoholic Fatty Liver Disease". Annals of Internal Medicine. 143 (10): 722–728. doi:10.7326/0003-4819-143-10-200511150-00009. PMID 16287793. S2CID 22475943.
- ^ Sarah Boseley (12 April 2019). "Experts warn of fatty liver disease 'epidemic' in young people". The Guardian. Retrieved 4 July 2023.
- ^ SPINK HEALTH (11 April 2019). "Nonalcoholic fatty liver disease found in large numbers of teenagers and young adults". EurekAlert! (Press release). American Association for the Advancement of Science. Retrieved 4 July 2023.
- ^ Cinque F, Cespiati A, Lombardi R, Costantino A, Maffi G, Alletto F, et al. (January 2022). "Interaction between Lifestyle Changes and PNPLA3 Genotype in NAFLD Patients during the COVID-19 Lockdown". Nutrients. 14 (3): 556. doi:10.3390/nu14030556. ISSN 2072-6643. PMC 8838646. PMID 35276911.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Liu, Y.L.; Patman, G.L.; Leathart, J.B.; Piguet, A.C.; Burt, A.D.; Dufour, J.F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75–81.
- ^ Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279.
- ^ Dutta D, Nagendra L, Mohindra R, Bhattacharya S, Joshi A, Kamrul-Hasan A (2024). "Role of Growth Hormone Therapy in Metabolic-Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis". Indian Journal of Endocrinology and Metabolism. 28 (4): 336–342. doi:10.4103/ijem.ijem_488_23. PMC 11451958. PMID 39371653.
- ^ Lock B (8 August 2017). "Hepatic Lipidosis (Fatty Liver Disease) in Reptiles". Vin.com. Retrieved 29 December 2020.
- ^ "Fatty Liver Disease in Birds". Animal House of Chicago. Retrieved 29 December 2020.
- ^ "Fatty Liver Disease in Cats". PetMD. Retrieved 29 December 2020.
- ^ Kalyesubula M, Mopuri R, Rosov A, Alon T, Edery N, Moallem U, et al. (December 2020). "Hyperglycemia-stimulating diet induces liver steatosis in sheep". Scientific Reports. 10 (1): 12189. Bibcode:2020NatSR..1012189K. doi:10.1038/s41598-020-68909-z. PMC 7376193. PMID 32699301.
- ^ Kalyesubula M, Mopuri R, Asiku J, Rosov A, Yosefi S, Edery N, et al. (1 March 2021). "High-dose vitamin B1 therapy prevents the development of experimental fatty liver driven by overnutrition". Disease Models & Mechanisms. 14 (3): dmm048355. doi:10.1242/dmm.048355. PMC 7988776. PMID 33608323.
External links
[edit]- Photo at Atlas of Pathology
